Abstract
Since ancient times, Coccinia grandis has been utilized for its numerous health benefits. Furthermore, it provides nutritional and therapeutic benefits without being harmful. Coccinia grandis, a member of the Cucurbitaceae family, has been used traditionally to treat metabolic syndrome and skin conditions. Because this plant contains a variety of chemical constituents, such as phenolic acid, flavonoids, terpenoids, alkaloids, glycosides, carotenoids, sterols, and coumarins, among others, researchers have determined through various animal studies that different parts of this plant exhibit desirable pharmacological effects, including antidiabetic, anti-hyperlipidemic, antioxidant, and hematopoietic effects.
Cite
- MLA: Md Maksud Ur Rahman, Aka Barua Joya, Md. Shafayat Hossain, Sumiya Binta Zaman, Md Tayeb Hossen, Tahasin Akther, Hridoy Chandra Ghosh, Suplob mandal, Junayed Alauddin, Md Ashraful Alam, Nusrat Subhan. "A Review on the Antidiabetic, Antioxidant, Antidyslipidemic, and Hepatoprotective Effects of Coccinia grandis ." J. Bio. Exp. Pharm 3.1 (2025): 20-37.
- APA: Md Maksud Ur Rahman, Aka Barua Joya, Md. Shafayat Hossain, Sumiya Binta Zaman, Md Tayeb Hossen, Tahasin Akther, Hridoy Chandra Ghosh, Suplob mandal, Junayed Alauddin, Md Ashraful Alam, Nusrat Subhan, (2025). A Review on the Antidiabetic, Antioxidant, Antidyslipidemic, and Hepatoprotective Effects of Coccinia grandis . J. Bio. Exp. Pharm, 3(1), 20-37.
- Chicago: Md Maksud Ur Rahman, Aka Barua Joya, Md. Shafayat Hossain, Sumiya Binta Zaman, Md Tayeb Hossen, Tahasin Akther, Hridoy Chandra Ghosh, Suplob mandal, Junayed Alauddin, Md Ashraful Alam, Nusrat Subhan. "A Review on the Antidiabetic, Antioxidant, Antidyslipidemic, and Hepatoprotective Effects of Coccinia grandis ." J. Bio. Exp. Pharm 3, no. 1 (2025): 20-37.
- Harvard: Md Maksud Ur Rahman, Aka Barua Joya, Md. Shafayat Hossain, Sumiya Binta Zaman, Md Tayeb Hossen, Tahasin Akther, Hridoy Chandra Ghosh, Suplob mandal, Junayed Alauddin, Md Ashraful Alam, Nusrat Subhan, 2025. A Review on the Antidiabetic, Antioxidant, Antidyslipidemic, and Hepatoprotective Effects of Coccinia grandis . J. Bio. Exp. Pharm, 3(1), pp.20-37.
- Vancouver: Md Maksud Ur Rahman, Aka Barua Joya, Md. Shafayat Hossain, Sumiya Binta Zaman, Md Tayeb Hossen, Tahasin Akther, Hridoy Chandra Ghosh, Suplob mandal, Junayed Alauddin, Md Ashraful Alam, Nusrat Subhan. A Review on the Antidiabetic, Antioxidant, Antidyslipidemic, and Hepatoprotective Effects of Coccinia grandis . J. Bio. Exp. Pharm. 2025;3(1):20-37.
Keywords
1. Introduction
A member of the Cucurbitaceae family, Coccinia grandis (L.) is a perennial climbing vine that grows quickly (2). Additionally, Coccinia grandis is an East African native plant that has spread by seed to various parts of tropical Asia and the Pacific region (3). Not only is this plant used in traditional medicine extensively but also as a nutritious vegetable. Almost any part of the plant has been used as a traditional medicine in Asian countries to treat skin diseases such as leprosy, acne, scabies, and wounds. It has also been used to treat poisoning, malaria, jaundice, and hepatitis, and it is now traditionally used to treat diabetes and obesity (4). Moreover, In Southeast Asia, the squashed fresh leaves of this plant are used as a traditional herbal treatment for bruises and itching from bug bites. This process is done by applying the crushed leaves directly to the lesion. So, it was also added as one of the traditional home remedies used to treat common illnesses (5). The whole plant of Coccinia grandis having pharmacological activities like analgesic, antipyretic, anti-inflammatory, antimicrobial, antiulcer, antidiabetic, antioxidant, hypoglycemic, hepatoprotective, antimalarial, antidyslipidemic, anticancer, antitussive, mutagenic and the present review gives botany, chemical constituents, and pharmacological activities of Coccinia grandis (6, 7). For individuals recently diagnosed with type 2 diabetes mellitus, an herbal medication made from Coccinia grandis extract showed promise as a helpful treatment. The medication was safe, well tolerated, and greatly improved glycemic and lipid profile parameters in randomized and controlled clinical trials (8). Evidences also suggest that the plant's ethanolic extract may be safely incorporated into herbal remedies for the clinical development of antibacterial activity against strains of bacteria that are resistant to many drugs (3). This review focuses on the pharmacological responses of the sections of the Coccinia grandis plant in this study, which include hepatoprotective, antioxidant, antihyperlipidemic, and antidiabetic activities.
2. Morphological Characteristics
2.1 Synonyms of Coccinia grandis
Many names are associated with Coccinia grandis, including Staphylosyce, Physedra, Cephalandra indica, Coccinia indica, and Coccinia cordifolia. It is known by various names, including kundru (Urdu), tindoli (Oriya), ivy gourd (English), and scarlet gourd. (9-11) In Bangladesh, it is known as Telakucha (12).
2.2 Taxonomy and visual inspection
Kingdom: Plantae; Order: Cucurbitales; Family: Cucurbitaceae; Genus: Coccinia; Species: Coccinia grandis (L.) (13, 14)
Figure 1: Different parts of Coccinia grandis (L.) Voigt
2.3 Distribution of Coccinia grandis
In different subtropical and tropical places of the world, as garden vegetables, sometimes Coccinia grandis (Ivy gourd) is cultivated. In the case of Asia, India, and central Africa, it is native. In the case of its base, which humans have obscured, there are also some important aspects of the process that are available, such as transportation, cultivation, usage, and history. In the case of Southeast Asia, it is considered a common weed. In various countries, such as the Solomon Islands, Marshall Islands, Guam, Fiji, Vanuatu, Hawaii, Florida, and Texas, it is neutralized. While in India and Southeast Asia by the local people, it is known as a valuable wild vegetable (32).
2.4 Botanical description of Coccinia grandis
This plant is recognized for its significant morphological variation among wild accessions and cultivated varieties (33).
Table 1: Different parts of the plants with their description
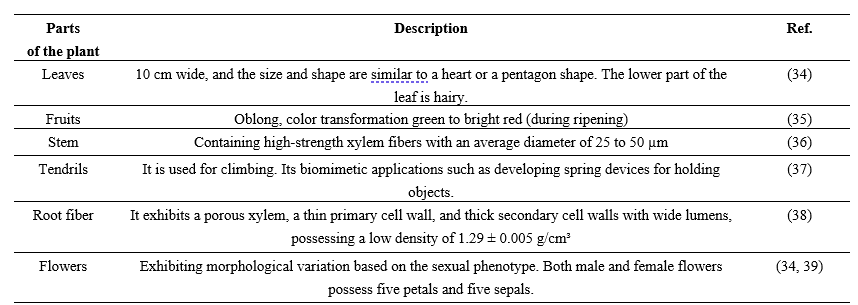
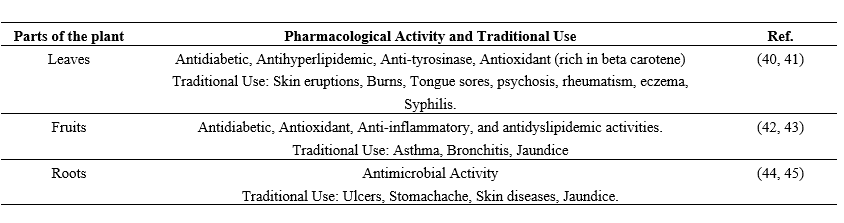
Table 2: Different parts of Coccinia grandis plants, and their medicinal use
3. Phytochemical and Chemical Constituents
3.1 Phytochemical Composition of Coccinia grandis
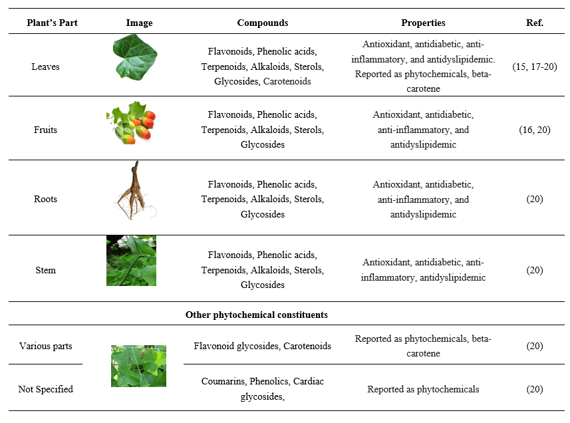
The pharmacological properties of Coccinia grandis are attributed to its abundance of bioactive substances, such as triterpenes, amino acids, flavonoids, and phenolics(15, 16).
Table 3: Different parts of the plant Coccinia grandis and their compounds, with their properties
3.2 Nutrient composition of Coccinia grandis
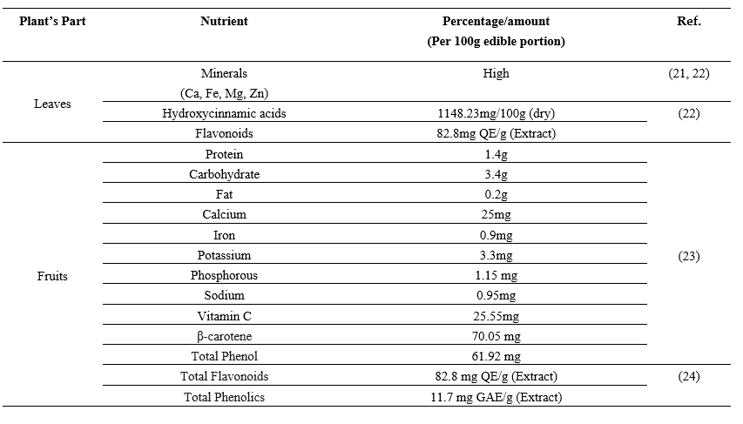
Table 4: Parts of Coccinia grandis with their nutrient compositions
3.3 Chemical constituents of Coccinia grandis
Table 5: Parts of Coccinia grandis with their phytochemical constituents
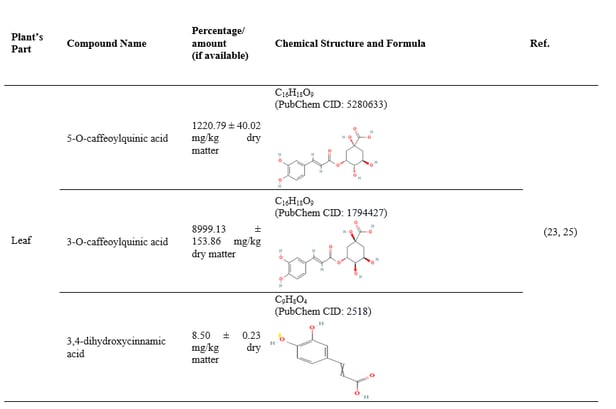
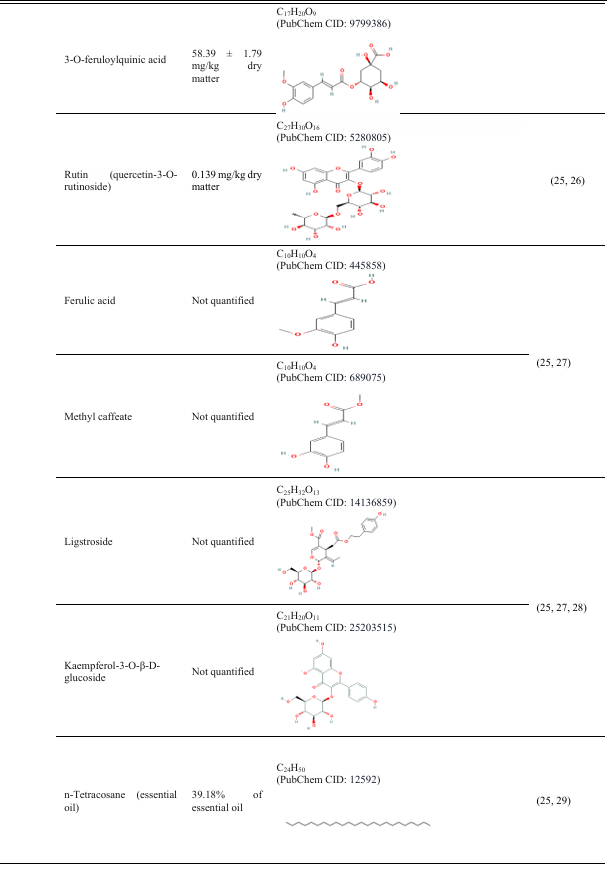
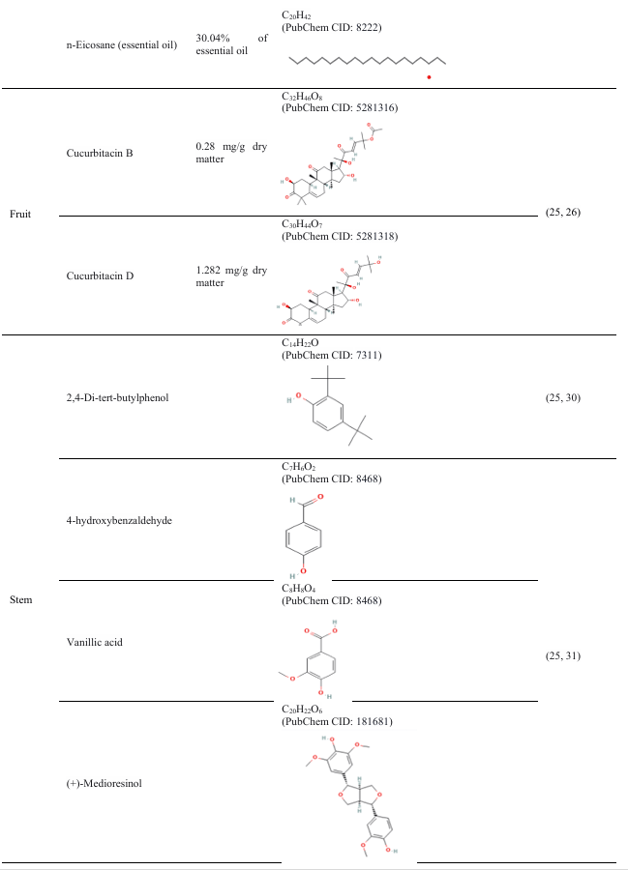
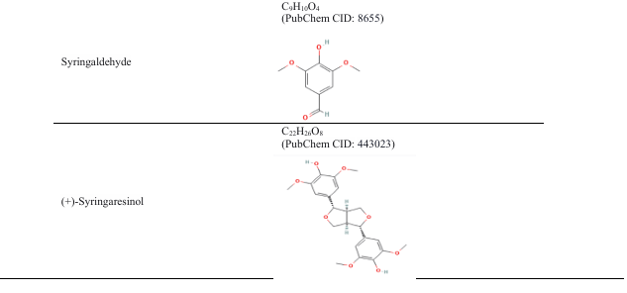
4. Therapeutic response of Coccinia grandis
4.1 Antidiabetic effect
To comprehend the antidiabetic action of Coccinia grandis, it is essential to understand the fundamental pharmacological mechanism of an animal model of diabetes. All of the data pertaining to beta cell malfunction, insulin resistance, and two major risk factors for diabetes mellitus have been compiled. The liver, muscle, and fat cells are less responsive to insulin, particularly in individuals with Type 2 diabetes mellitus (T2DM). This reduces the body's ability to absorb glucose, causing the liver to continually produce glucose even when the body doesn't need it. Insulin resistance is caused by a variety of factors, including genetics, obesity, chronic inflammation, excess fatty acids, and dysregulated adipokines (46, 47). The inability of the pancreatic beta cells to perform their intended tasks may result in the release of sufficient insulin to overcome resistance. Beta cells are further harmed by lipo-toxicity and glucose toxicity, which facilitate their degradation (47, 48). The normal insulin pathway is depicted in Figure 2 .
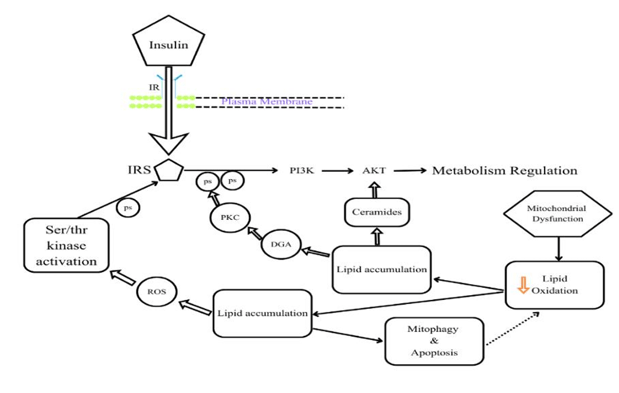
Figure 2: Normal Insulin Signaling Pathway in this figure In this diagram, IR- Insulin Receptor, IRS- Insulin Receptor Substrate, PKc- Protein Kinase C, ROS- Reactive Oxygen Species, Ps- Phosphorylation, DAG Diacylglycerols, PI3K-Phosphoinositide-3-kinase (1).
Table 5: The key factors in insulin resistance and beta-cell dysfunction

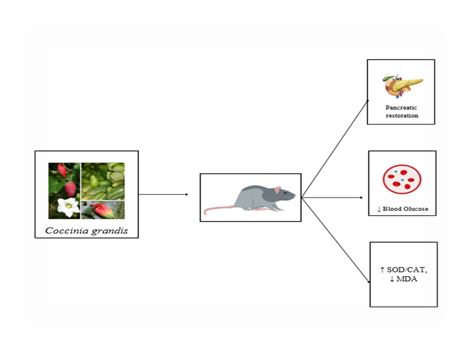
Figure 3: Antidiabetic effect of Coccinia grandis
4.2 Antihyperlipidemic effect
The term ‘hyperlipidemia’ refers to the condition characterized by abnormally high levels of lipids in the human systemic circulation, primarily triglycerides (TG) and cholesterol. The body produces, absorbs, transports, and eliminates lipoproteins, which include chylomicrons, VLDL, LDL, and HDL, in an imbalanced way. High dietary fat intake, heredity, and insulin resistance are among the variables that influence cholesterol homeostasis. This imbalance may promote cardiovascular disease and result in the development of atherosclerosis (62-65). After eating a high-fat meal that has a lot of TGs, chylomicrons, and VLDL, the amount of lipoprotein goes up. In hyperlipidemic situations, these lipoproteins take longer to clear, which leads to a persistent rise in the number of particles in the blood(62, 65). VLDL and chylomicron residual balance depend on lipoprotein lipase activity. The primary role of lipoprotein lipase activity is compromised in cases of metabolic syndrome development, such as obesity and type 2 diabetes. Therefore, hyperlipidemia is a possibility (62, 63). Chronic inflammation and oxidative stress lead to the disruption of lipid metabolism and the development of endothelial dysfunction (64, 66-68).
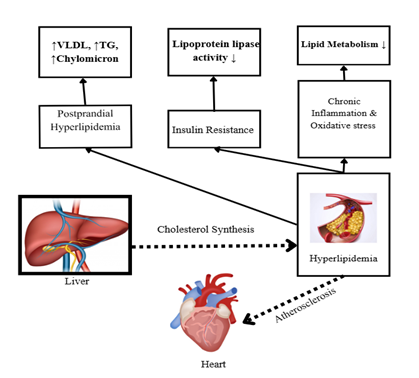
Figure 4: Mechanisms that are driving heart disease through the development of dyslipidemia.

According to research based on animal trials, Coccinia grandis extract exhibits antihyperlipidemic properties that can lower body weight and cholesterol levels (including triglycerides, VLDL, and LDL), while reducing the risk of vascular atherosclerosis.
Table 7: Evidence from animal studies is gathered to understand the effect of Coccinia grandis as an antidiabetic and antihyperlipidemic agent.
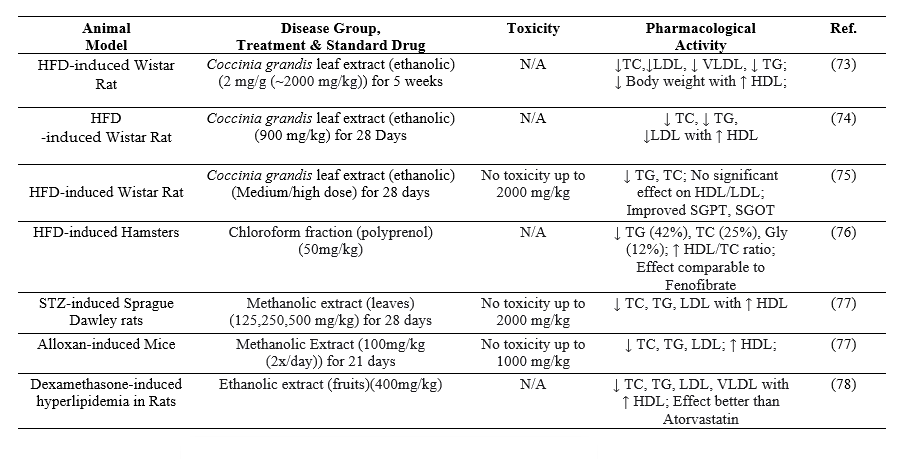

Figure 5: Antihyperlipidemic effect of Coccinia grandis.
4.3 Antioxidant effect
When the body produces more free radicals than it can counteract, primarily reactive oxygen species (ROS) and reactive nitrogen species (RNS), the state is known as oxidative stress. Both endogenous (normal physiological processes) and exogenous (external exposures) pathways may produce these free radicals. The mitochondrial electron transport chain generates highly reactive hydroxyl radicals (•OH) during the process of ATP synthesis. During the synthesis of ATP, superoxide (O2•) is first produced and then radicals (into hydrogen peroxide (H2O2). Extremely reactive hydroxyl free radicals are produced by the Fenton reaction.(79-81). Numerous studies indicate that several enzymes, including nitric oxide synthase, cytochrome P450, xanthine oxidase, and NADPH oxidase, may be involved in the production of ROS and RNS during immunological and metabolic processes (79-81). The generation of lipid radicals and peroxyl radicals can be produced due to the initiation of the chain reaction by abstracting hydrogen from polyunsaturated fatty acids in membranes(79, 80). During a respiratory burst can be produced a large number of ROS/RNS during the activation of immune cells, including macrophages and neutrophils, to destroy pathogens (79, 81-83). Several external environmental factors are responsible for stimulating the production of free radicals in the body, including Ultraviolet and ionizing radiation, pollution, cigarette smoke, certain drugs, and heavy metals. These factors can generate free radicals or stimulate their production in tissues. (79, 81, 82).
According to some research-based data, Coccinia grandis extract contains antioxidant properties that can help lower the body's oxidative stress levels by reducing the formation of highly reactive oxygen and nitrogen species.
Table 8: The antioxidant activity of C. grandis in multiple animal model studies:
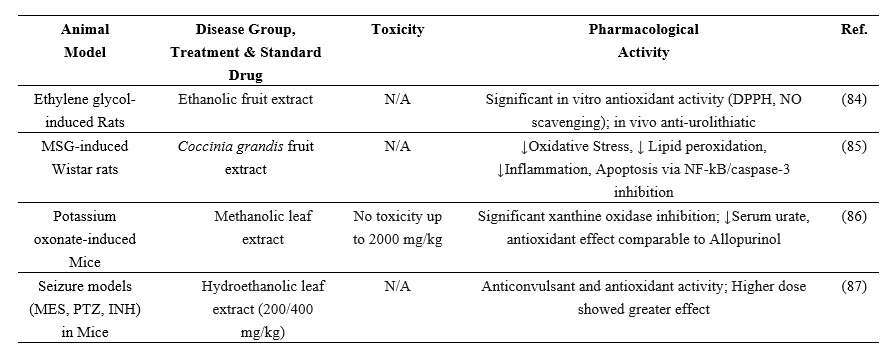
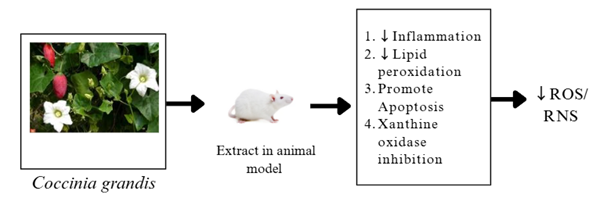
Figure 6: Antioxidant effect of Coccinia grandis.
4.4 Hepatoprotective effect
Multiple mechanisms, including direct injury, inflammation, and metabolic stress, may cause damage to hepatocytes. The primary processes that harm hepatocytes and result in fibrosis and inflammation include apoptosis, necrosis, pyroptosis, and organelle dysfunctions. The most widely used biomarker for liver function is the measurement of serum enzyme levels; however, other specialized biomarkers are increasingly available. The damage in hepatocytes may occur due to apoptosis (regulated cell death), necrosis (uncontrolled lysis), and pyroptosis (inflammatory cell death). The release of inflammasome particles is able to activate hepatic stellate cells and further drive them to fibrosis in the case of pyroptosis (88, 89)The production of DAMPs, or damage-associated molecular patterns, may occur in hepatocytes when certain essential organelles, including the mitochondria, lysosomes, and endoplasmic reticulum, sustain damage. These DAMPs have the ability to cause hepatocyte inflammation and further damage(89, 90).Cytokines, chemokines, and extracellular vesicles are released by a damaged hepatocyte, increasing inflammation and attracting immune cells. Additionally, this may stimulate hepatic stellate cells, which could lead to fibrosis (88, 89, 91, 92).Hepatic cell damage is caused by a variety of factors, including autoimmune illnesses, metabolic abnormalities, ischemia/reperfusion, alcohol use, viral hepatitis, and medications like paracetamol (89, 91).
Table 9: Biomarkers to understand liver function are given below
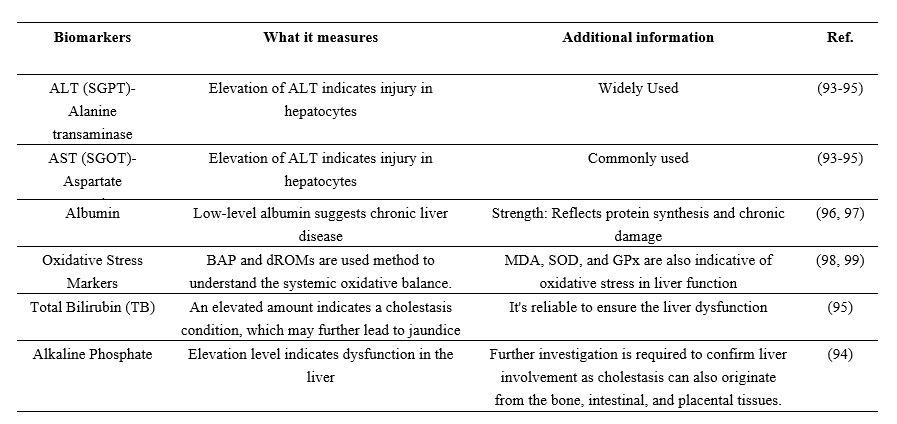
Table 10: Hepatoprotective effects of Coccinia grandis in animal models
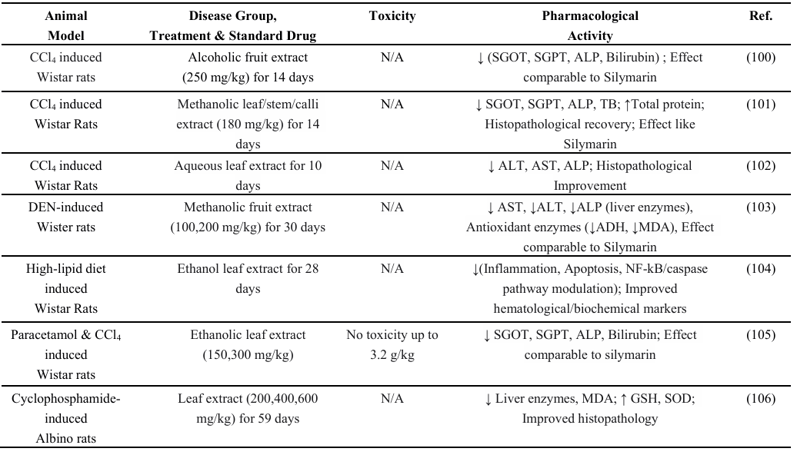
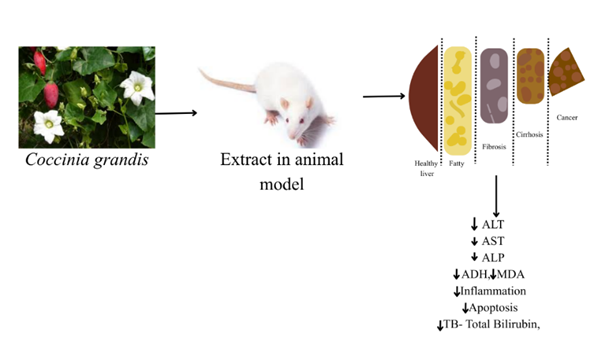
According to research on animal models, extracts from various parts of Coccinia grandis can lower several indicators related to liver function, providing insight into the plant's hepatoprotective effects (Table 10). It has the capacity to lower hepatocyte inflammation and apoptosis, as well as oxidative indicators MDA and SOD, which are closely related to liver function.
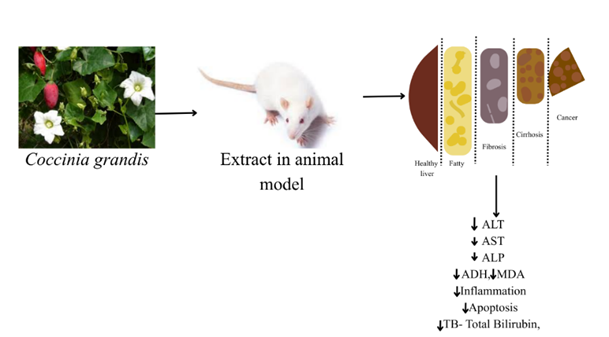
Figure 7: Hepatoprotective effect of Coccinia grandis.
5. Conclusion
Author Contribution
Conceptualization, MAA and NS; methodology, MAA, MMUR, ABJ, JA; formal analysis, MSH, SBZ, TA, HCG; investigation, MSH, SBZ, TA, MTH; resources, ABJ, HCG, JA; data curation, MMUR, HCG; writing—original draft preparation, MMUR, MSH, SBZ; writing—review and editing, MMUR, HCG, JA; visualization, MAA, NS; supervision, MAA, NS; project administration, NS; All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any internal or external funding from profit and non-profit organization.
Institutional Review Board Statement
Not Applicable (N/A)
Data Availability Statement
Data used in this study will be available upon reasonable request from the corresponding author.
Acknowledgments
Authors are gratefully acknowledging the logistic support from the department of Pharmaceutical Sciences, North South University.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
-
Chandrasekaran, P.; Weiskirchen, R. Cellular and Molecular Mechanisms of Insulin Resistance. Curr. Tissue Microenviron. Rep. 2024, 5(3), 79-90. https://doi.org/10.1007/s43152-024-00056-3
-
Hossain, M. S.; Jahan, I.; Islam, M.; Nayeem, J.; Anzum, T. S.; Afrin, N. A.; Mim, F. K.; Hasan, M. K. Coccinia grandis: Phytochemistry, pharmacology and health benefits. Clin. Tradit. Med. Pharmacol. 2024, 5(2), 200150. https://doi.org/10.1016/j.ctmp.2024.200150
-
Alshahrani, M. Y.; Ibrahim, E. H.; Asiri, M.; Kilany, M.; Alshehri, A.; Alkhathami, A. G.; Alshahrani, M. Inhibition realization of multidrug resistant bacterial and fungal isolates using Coccinia indica extracts. Saudi J. Biol. Sci. 2022, 29(5), 3207-3212. https://doi.org/10.1016/j.sjbs.2022.01.045
-
Chuchote, C.; Somwong, P. Assessment of the ethanolic extract of Coccinia grandis on in vitro anti-tyrosinase and anti-inflammatory activities and its active chemical determination. Food Res. 2024, 8(4), 162-169. https://doi.org/10.26656/fr.2017.8(4).427
-
Namchaiw, P.; Jaisin, Y.; Niwaspragrit, C.; Malaniyom, K.; Auvuchanon, A.; Ratanachamnong, P. The Leaf Extract of Coccinia grandis (L.) Voigt Accelerated In Vitro Wound Healing by Reducing Oxidative Stress Injury. Oxid. Med. Cell. Longev. 2021, 2021(1), 3963510. https://doi.org/10.1155/2021/3963510
-
Siddiqua, S.; Jyoti, F. H.; Saffoon, N.; Miah, P.; Lasker, S.; Hossain, H.; Akter, R.; Ahmed, M. I.; Alam, M. A. Ethanolic extract of Coccinia grandis prevented glucose intolerance, hyperlipidemia and oxidative stress in high fat diet fed rats. Phytomed. Plus. 2021, 1(4), 100046. https://doi.org/10.1016/j.phyplu.2021.100046
-
Sakharkar, P.; Chauhan, B. Antibacterial, antioxidant and cell proliferative properties of Coccinia grandis fruits. Avicenna J. Phytomed. 2017, 7(4), 295-307.
-
Wasana, K. G. P.; Attanayake, A. P.; Weerarathna, T. P.; Jayatilaka, K. A. P. W. Efficacy and safety of a herbal drug of Coccinia grandis (Linn.) Voigt in patients with type 2 diabetes mellitus: A double blind randomized placebo controlled clinical trial. Phytomedicine. 2021, 81, 153431. https://doi.org/10.1016/j.phymed.2020.153431
-
Yadav, L. P.; Gangadhara, K.; Apparao, V. V.; Yadav, V.; Mishra, D. S.; Singh, A. K.; Rane, J.; Kaushik, P.; Janani, P.; Kumar, R.; Verma, A. K.; Kumar, S.; Malhotra, S. K.; Shekhawat, N. Genetic diversity, morphological traits, quality traits and antioxidants potentiality of Coccinia grandis germplasm under rainfed semi-arid region. Sci. Rep. 2024, 14(1), 868. https://doi.org/10.1038/s41598-023-49091-4
-
Sarkar, T.; Mukherjee, A.; Chatterjee, K.; Akhmetova, S. O.; Alipbekova, A. S.; Temerbayeva, M.; Rebezov, M.; Shariati, M. A.; Lorenzo, J. M. Quality Assessment of tindora (Coccinia indica) using poincare plot and cartesian quadrant analysis. Food Anal. Methods. 2022, 15(9), 2357-2371. https://doi.org/10.1007/s12161-022-02287-2
-
Saikia, J.; Phookan, D.; Talukdar, P. Studies on genetic variability in ivy gourd [Coccinia grandis (L.) Voigt.]. Indian J. Hortic. 2017, 74(1), 139-141. https://doi.org/10.5958/0974-0112.2017.00031.7
-
Sarkar, S. K.; Uddin, M. M.; Hossain, M. M.; Masum, M. A.; Islam, M. S. Hematobiochemical effects of Telakucha (Coccinia indica) in alloxan induced diabetic rats. Res. Agric. Livest. Fish. 2020, 7(3), 431-438. https://doi.org/10.3329/ralf.v7i3.51362
-
Rahman, M. S.; Asaduzzaman, M.; Munira, S.; Rahman, M. M.; Hasan, M.; Siddique, A. H.; Khatun, M.; Islam, M. A. Evaluation of phytochemical, antibacterial, antioxidant, and cytotoxic properties of Coccinia cordifolia leaves. Int. J. Adv. Res. 2015, 3(8), 384-394.
-
Bussmann, R. W.; Paniagua-Zambrana, N. Y.; Njoroge, G. N. Coccinia grandis (L.) Voigt Cucurbitaceae. Ethnobot. Mt. Reg. 2021, 309-311. https://doi.org/10.1007/978-3-030-38386-2_44
-
Putra, I. M. W. A.; Kusumawati, I. G. A. W.; Sumadewi, N. L. U. Physical Characteristics, Total Phenolic, and Flavonoid Content of Coccinia grandis (L.) Voigt Leaves Extract. Acta Chim. Asiana. 2021, 4(2), 114-119. https://doi.org/10.29303/aca.v4i2.66
-
Lee, I. Y.; Park, J. H.; Joo, N. Changes in free amino acid, phenolic, triterpene and biogenicamine contents in Coccinia grandis pickles during aging. J. Agric. Food Res. 2024, 15, 101000. https://doi.org/10.1016/j.jafr.2024.101000
-
Shanmuganathan, E.; Arawwawala, L. D. A. M.; Wasana, K. G. P.; Attanayake, A. P. Selection and optimisation of extraction technique for the preparation of phenolic- and flavonoid-rich extract of leafy vegetable, Coccinia grandis (Linn.) Voigt. Int. Food Res. J. 2022, 29(5), 1032-1042. https://doi.org/10.47836/ifrj.29.5.06
-
Ahmed, R. M.; Widdatallah, M. O.; Alrasheid, A. A.; Widatallah, H. A.; Alkhawad, A. O. Evaluation of the phytochemical composition, antioxidant properties, and in vivo antihyperglycemic effect of Coccinia grandis leaf extract in mice. Univers. J. Pharm. Res. 2024, 9(2). https://doi.org/10.22270/ujpr.v9i2.1091
-
Prabhakar, P.; Mukherjee, S.; Kumar, A.; Rout, R. K.; Kumar, S.; Verma, D. K.; Dhara, S.; Rao, P. S.; Maiti, M. K.; Banerjee, M. In silico, In vitro and Ex vivo Assessment of Antihyperglycemic, Antioxidant and Cytotoxic Properties of Ivy Gourd (Coccinia grandis L.) Leaf Extract. Food Technol. Biotechnol. 2024, 62(2), 188-204. https://doi.org/10.17113/ftb.62.02.24.8162
-
Lee, I. Y.; Lee, D. H.; Park, J. H.; Joo, N. UHPLC-HRMS/MS–Based Metabolic Profiling and Quantification of Phytochemicals in Different Parts of Coccinia grandis (L.) Voigt. Food Sci. Nutr. 2025, 13(2). https://doi.org/10.1002/fsn3.70004
-
Preetha, T. S. Heavy metal screening of Coccinia grandis (L). Voigt leaves using atomic absorption spectroscopy (AAS). Int. J. Sci. Res. 2020, 9(3), 33-35.
-
Moulick, S. P.; Jahan, F.; Mamun, M. Z. U. A.; Hossain, M. I. S.; Ahmed, K. S.; Kabir, M. A.; Rashid, M. M.; Sathee, R. A.; Hasan, M. M. Boerhavia diffusa and Coccinia grandis: Two Indigenous vegetables as a source of essential minerals, vitamins, amino acids, and fatty acids. Appl. Food Res. 2024, 4(2), 100494. https://doi.org/10.1016/j.afres.2024.100494
-
Neetu; Purwar, S.; Bisht, V.; Neeraj; Maurya, B. Nutritional and therapeutic values of Coccinea grandis: A review. Int. J. Chem. Stud. 2020, 8(4), 2528-2534. https://doi.org/10.22271/chemi.2020.v8.i4o.9832
-
Kondhare, D.; Lade, H. Phytochemical profile, aldose reductase inhibitory, and antioxidant activities of Indian traditional medicinal Coccinia grandis (L.) fruit extract. 3 Biotech. 2017, 7, 1-10. https://doi.org/10.1007/s13205-017-1013-1
-
National Center for Biotechnology Information. PubChem Compound Summary. PubChem. 2021.
-
Lee, I. Y.; Joo, N. Identification and Quantification of Key Phytochemicals, Phytohormones, and Antioxidant Properties in Coccinia grandis during Fruit Ripening. Antioxidants 2022, 11(11), 2218. https://doi.org/10.3390/antiox11112218
-
Al-Madhagy, S.; Mostafa, N.; Youssef, F.; Awad, G.; Eldahshan, O.; Singab, A. Isolation and structure elucidation of compounds from Coccinia grandis leaves extract. Egypt. J. Chem. 2019, 62(10), 1891-1903. https://doi.org/10.21608/ejchem.2019.10925.1700
-
Al-Madhagy, S.; Mostafa, N.; Youssef, F.; Awad, G.; Eldahshan, O.; Singab, A. Metabolic profiling of a polyphenolic-rich fraction of Coccinia grandis leaves using LC-ESI-MS/MS and in vivo validation of its antimicrobial and wound healing activities. Food Funct. 2019, 10(10), 6667-6674. https://doi.org/10.1039/C9FO01532A
-
Mohammed, S.; Vishwakarma, K.; Maheshwari, V. Evaluation of Larvicidal Activity of Essential Oil from Leaves of Coccinia grandis against Three Mosquito Species. J. Arthropod Borne Dis. 2017, 11(2), 226-235.
-
Momin, Y. H.; Yeligar, V. C.; Saralaya, M. G.; Dharmamoorthy, G.; Mallikarjuna, B. P.; Jadhav, S.; Das, K.; Almuqbil, M.; Ahmad, F.; Rabbani, S. I.; Asdaq, S. M. B . Computational investigation of 2, 4-Di Tert Butyl Phenol as alpha amylase inhibitor isolated from Coccinia grandis (L.) Voigt using molecular docking, and ADMET parameters. Comput. Biol. Chem. 2024, 110, 108087. https://doi.org/10.1016/j.compbiolchem.2024.108087
-
Trinh, P. T. P.; Nguyen, T. T.; Van Hau, N.; Hung, Q. T.; Du, C. V.; Tuan, N. T.; Thao, N. P. Chemical constituents of the stem of Coccinia grandis. IOP Conf. Ser. Mater. Sci. Eng. 2020, 736(2), 022080. https://doi.org/10.1088/1757-899X/736/2/022080
-
Wasantwisut, E.; Viriyapanich, T. Ivy gourd (Coccinia grandis Voigt, Coccinia cordifolia, Coccinia indica) in human nutrition and traditional applications. World Rev. Nutr. Diet. 2003, 91, 60-66. https://doi.org/10.1159/000069929
-
Shaina, T. J.; Beevy, S. S. Morphological variation and evolutionary significance of Coccinia grandis (L.) Voigt: an under-exploited cucurbitaceous vegetable crop. Plant Syst. Evol. 2012, 298(3), 653-659. https://doi.org/10.1007/s00606-011-0574-4
-
Devani, R. S.; Sinha, S.; Banerjee, J.; Sinha, R. K.; Bendahmane, A.; Banerjee, A. K. De novo transcriptome assembly from flower buds of dioecious, gynomonoecious and chemically masculinized female Coccinia grandis reveals genes associated with sex expression and modification. BMC Plant Biol. 2017, 17(1), 241. https://doi.org/10.1186/s12870-017-1187-z
-
Ramachandran, K.; Subramaniam, B. Scarlet gourd Coccinia grandis little-known tropical drug plant. Econ. Bot. 1983, 37(4), 380-383. https://doi.org/10.1007/BF02904197
-
Jebadurai, S. G.; Raj, R. E.; Sreenivasan, V. S.; Binoj, J. S. Comprehensive characterization of natural cellulosic fiber from Coccinia grandis stem. Carbohydr. Polym. 2019, 207, 675-683. https://doi.org/10.1016/j.carbpol.2018.12.027
-
Prombanchong, T.; Suriyawong, A.; Srisongkram, P.; Suwanpayak, N. Anatomical and physical properties of tendril perversion of Coccinia grandis (L.) Voigt. IOP Conf. Ser. Mater. Sci. Eng. 2020, 965(1), 012011. https://doi.org/10.1088/1757-899X/965/1/012011
-
Jebadurai, S. G.; Raj, R. D. E.; Sreenivasan, V. S. Characterization of Natural Cellulosic Fiber from Coccinia grandis Root. J. Nat. Fibers 2022, 19(14), 9444-9456. https://doi.org/10.1080/15440478.2021.1982833
-
Ghadge, A. G.; Karmakar, K.; Devani, R. S.; Banerjee, J.; Mohanasundaram, B.; Sinha, R. K.; Sinha, S.; Banerjee, A. K. Flower development, pollen fertility and sex expression analyses of three sexual phenotypes of Coccinia grandis. BMC Plant Biol. 2014, 14, 325. https://doi.org/10.1186/s12870-014-0325-0
-
Rahman, M.; Sarker, J.; Akter, S.; Mamun, A.; Azad, M.; Mohiuddin, M.; Shahid, I. Z. Comparative evaluation of antidiabetic activity of crude methanolic extract of leaves, fruits, roots and aerial parts of Coccinia grandis. J. Plant Sci. 2015, 2(6-1), 19-23.
-
Lobo, J. A.; Sowmya, S. Pharmacological Review on Coccinia grandis Leaves. Int. J. Pharm. Sci. Rev. Res. 2022, 74(2), 96-99. https://doi.org/10.47583/ijpsrr.2022.v74i02.014
-
Lee, I. Y.; Joo, N. Identification and Quantification of Key Phytochemicals, Phytohormones, and Antioxidant Properties in Coccinia grandis during Fruit Ripening. Antioxidants 2022, 11(11), 2218. https://doi.org/10.3390/antiox11112218
-
Mandal, D.; Majumder, S.; Shah, D. J.; Guha, S.; Das, M.; Karmakar, A.; Seal, R. Coccinia grandis a Potential Herb in Traditional Use and Recent Established Biological Activities with New Prospectives - A Review. Trop. J. Pharm. Life Sci. 2025, 12(3), 183. https://doi.org/10.61280/tjpls.v12i3.183
-
Hasanuzzaman, M.; Sayeed, M. S. B.; Islam, M. S.; Sarwar, M. S.; Moghal, M. M. R.; Ahmed, J. U.; Islam, M. A. Preliminary antimicrobial activity and cytotoxicity of plant extracts (roots) of Coccinia grandis (Family: Cucurbitaceae). Int. J. Pharm. Sci. Res. 2013, 4(4), 1466-1470.
-
Neetu; Purwar, S.; Bisht, V.; Neeraj; Maurya, B. K. R. Nutritional and therapeutic values of Coccinea grandis: A review. Int. J. Chem. Stud. 2020, 8(4), 1555-1561. https://doi.org/10.22271/chemi.2020.v8.i4o.9832
-
Kumari, N.; Radha; Kumar, M.; Mekhemar, M.; Lorenzo, J. M.; Pundir, A.; Devi, K. B.; Prakash, S.; Puri, S.; Thakur, M.; Rathour, S.; Rais, N.; Jamwal, R.; Kumar, A.; Dhumal, S.; Singh, S.; Senapathy, M.; Dey, A.; Chandran, D.; Andrade-Cetto, A. Therapeutic uses of wild plant species used by rural inhabitants of Kangra in the western Himalayan region. S. Afr. J. Bot. 2022, 148, 415-436. https://doi.org/10.1016/j.sajb.2022.05.004
-
Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K. B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21(17), 6275. https://doi.org/10.3390/ijms21176275
-
Yang, Y.; Chen, Z.; Zhao, X.; Xie, H.; Du, L.; Gao, H.; Xie, C. Mechanisms of Kaempferol in the treatment of diabetes: A comprehensive and latest review. Front. Endocrinol. 2022, 13, 990299. https://doi.org/10.3389/fendo.2022.990299
-
Cerf, M. Beta Cell Dysfunction and Insulin Resistance. Front. Endocrinol. 2013, 4, 37. https://doi.org/10.3389/fendo.2013.00037
-
Kahn, S. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia 2003, 46, 3-19. https://doi.org/10.1007/s00125-002-1009-0
-
Khalid, M.; Alkaabi, J.; Khan, M.; Adem, A. Insulin Signal Transduction Perturbations in Insulin Resistance. Int. J. Mol. Sci. 2021, 22(16), 8590. https://doi.org/10.3390/ijms22168590
-
Oh, Y.; Bae, G.; Baek, D.; Park, E.-Y.; Jun, H. Fatty Acid-Induced Lipotoxicity in Pancreatic Beta-Cells During Development of Type 2 Diabetes. Front. Endocrinol. 2018, 9, 384. https://doi.org/10.3389/fendo.2018.00384
-
Rodríguez-Rodríguez, A.; Porrini, E.; Torres, A. Beta-Cell Dysfunction Induced by Tacrolimus: A Way to Explain Type 2 Diabetes? Int. J. Mol. Sci. 2021, 22(19), 10311. https://doi.org/10.3390/ijms221910311
-
Dinić, S.; Jovanović, J. A.; Uskoković, A.; Mihailović, M.; Grdović, N.; Tolić, A.; Rajić, J.; Đorđević, M.; Vidaković, M. Oxidative stress-mediated beta cell death and dysfunction as a target for diabetes management. Front. Endocrinol. 2022, 13, 1006376. https://doi.org/10.3389/fendo.2022.1006376
-
Made, I.; Putra, W. A.; Fakhrudin, N.; Nurrochmad, A.; Wahyuono, S. Antidiabetic effect of combined extract of Coccinia grandis and Blumea balsamifera on streptozotocin-nicotinamide induced diabetic rats. J. Ayurveda Integr. Med. 2024, 15(4), 101021. https://doi.org/10.1016/j.jaim.2024.101021
-
Meher, N.; Panda, B.; Ray, B. In Vivo Antidiabetic Activity of fruit extract of Coccinia grandis Linn in Normoglycemic, Adrenaline Induced and Alloxan Induced Diabetic rat. Res. J. Pharm. Technol. 2019, 12(11), 5143-5147. https://doi.org/10.5958/0974-360X.2019.01026.6
-
Gobinath, R.; Parasuraman, S.; Sreeramanan, S.; Sumitha, S. Antidiabetic and Antihyperlipidemic Activities of Methanolic Extract of Leaves of Coccinia grandis in Diabetic Rats. Int. J. Res. Pharm. Sci. 2020, 11(2), 2374-2379.
-
Mohammed, S.; Chopda, M.; Patil, R.; Vishwakarma, K.; Maheshwari, V. In vivo antidiabetic and antioxidant activities of Coccinia grandis leaf extract against streptozotocin induced diabetes in experimental rats. Asian Pac. J. Trop. Dis. 2016, 6(4), 298-304. https://doi.org/10.1016/S2222-1808(15)61034-9
-
Meenatchi, P.; Maneemegalai, S. Antidiabetic effect of Coccinia grandis Linn. on streptozotocin induced diabetic rats and its role in regulating hepatic key enzymes. J. Herb. Med. Toxicol. 2018, 12(1), 1-7.
-
Attanayake, A. P.; Jayatilaka, K. A. P. W.; Pathirana, C.; Mudduwa, L. K. B. Antihyperglycemic activity of Coccinia grandis (L.) Voigt in streptozotocin induced diabetic rats. S. L. J. Med. 2015, 24(1), 25-32.
-
Rahman, M.; Sarker, J.; Akter, S.; Mamun, A.; Azad, M.; Mohiuddin, M.; Haque, A. Comparative Evaluation of Antidiabetic Activity of Crude Methanolic Extract of Leaves, Fruits, Roots and Aerial Parts of Coccinia grandis. J. Plant Sci. 2015, 2(1), 19-23.
-
Yanai, H.; Adachi, H.; Hakoshima, M.; Katsuyama, H. Postprandial Hyperlipidemia: Its Pathophysiology, Diagnosis, Atherogenesis, and Treatments. Int. J. Mol. Sci. 2023, 24(17), 13118. https://doi.org/10.3390/ijms241713118
-
Maulana, H.; Ridwan, A. High-Fat Diets-Induced Metabolic Disorders to Study Molecular Mechanism of Hyperlipidemia in Rats. 3BIO: J. Biol. Sci. Technol. Manag. 2021, 3(2), 105-115. https://doi.org/10.5614/3bio.2021.3.2.5
-
Jia, X.; Xu, W.; Zhang, L.; Li, X.; Wang, R.-R.; Wu, S. Impact of Gut Microbiota and Microbiota-Related Metabolites on Hyperlipidemia. Front. Cell. Infect. Microbiol. 2021, 11, 634780. https://doi.org/10.3389/fcimb.2021.634780
-
Vergès, B. Intestinal lipid absorption and transport in type 2 diabetes. Diabetologia 2022, 65(10), 1587-1600. https://doi.org/10.1007/s00125-022-05765-8
-
Yao, Y.; Li, T.; Zeng, Z. Mechanisms underlying direct actions of hyperlipidemia on myocardium: an updated review. Lipids Health Dis. 2020, 19(1), 23. https://doi.org/10.1186/s12944-019-1171-8
-
Sun, J.; Du, B.; Chen, M.; Jia, J.; Wang, X.; Hong, J. FBXO28 reduces high-fat diet-induced hyperlipidemia in mice by alleviating abnormal lipid metabolism and inflammatory responses. J. Endocrinol. Invest. 2024, 47(10), 2445-2458. https://doi.org/10.1007/s40618-024-02376-5
-
Lai, M.; Peng, H.; Wu, X.; Chen, X.; Wang, B.; Su, X. IL-38 in modulating hyperlipidemia and its related cardiovascular diseases. Int. Immunopharmacol. 2022, 108, 108876. https://doi.org/10.1016/j.intimp.2022.108876
-
Feng, J.; Xu, R.; Dou, Z.; Hao, Y.; Xu, R.; Khoso, M. A.; Shi, Y.; Liu, L.; Sun, H.; Chen, C.; Li, X.; Liu, H.; Han, W.; Cheng, M.; Tang, P.; Li, J.; Zhang, Y.; Liu, X. Tetrahydroberberrubine improves hyperlipidemia by activating the AMPK/SREBP2/PCSK9/LDL receptor signaling pathway. Eur. J. Pharmacol. 2025, 988, 177228. https://doi.org/10.1016/j.ejphar.2024.177228
-
Hu, Y.; Xu, R.; Feng, J.; Zhang, Q.; Zhang, L.; Li, Y.; Chen, L. Identification of potential pathogenic hepatic super-enhancers regulatory network in high-fat diet induced hyperlipidemia. J. Nutr. Biochem. 2024, 127, 109584. https://doi.org/10.1016/j.jnutbio.2024.109584
-
Liang, X.; Zhang, Z.; Zhou, X.; Lu, Y.; Li, R.; Yu, Z.; Tong, L.; Gong. P.; Yi, H.; Zhang, L. Probiotics improved hyperlipidemia in mice induced by a high cholesterol diet via downregulating FXR. Food Funct. 2020, 11(11), 10134-10145. https://doi.org/10.1039/D0FO02255A
-
Duan, R.; Guan, X.; Huang, K.; Zhang, Y.; Li, S.; Xia, J.; Shen, M. Flavonoids from Whole-Grain Oat Alleviated High-Fat Diet-Induced Hyperlipidemia via Regulating Bile Acid Metabolism and Gut Microbiota in Mice. J. Agric. Food Chem. 2021, 69(27), 7618-7629. https://doi.org/10.1021/acs.jafc.1c01813
-
Hossain, M. F.; Rahaman, M. A.; Abdullah-Al-Mamun, H. K.; Islam, M. S.; Sumsuzzman, D. M. Antihyperlipidemic activity of Coccinia grandis on high fat diet induced wistar albino rats. Int. J. Res. Pharm. Biosci. 2017, 4(7), 15-20.
-
Toha, I. W.; Hasan, S. M. F.; Islam, S.; Ahmed, M. H.; Nobe, N.; Tasnim, S.; Shakil, F. S.; Chowdhury, M.M. Elucidation of Anti-hyperlipidemic Activity of 70% Ethanolic Extract of Coccinia grandis on High Fat-induced Hyperlipidaemic Wister Albino Rat Model. J. Complement. Altern. Med. Res. 2025, 26(3), 638. https://doi.org/10.9734/jocamr/2025/v26i3638
-
Nisu, R. A.; Nadvi, F. A.; Ankhi, A. A.; Suma, R. S.; Papiya, I. J.; Bose, A. An Evaluation of Anti-hyperlipidemic Activity of Ethanolic Extract of Coccinia grandis Leaves in High Fat Induced Rodent Model. Cardiol. Angiol. Int. J. 2024, 13(1), 387. https://doi.org/10.9734/ca/2024/v13i1387
-
Singh, G.; Gupta, P.; Rawat, P.; Puri, A.; Bhatia, G.; Maurya, R. Antidyslipidemic activity of polyprenol from Coccinia grandis in high-fat diet-fed hamster model. Phytomedicine 2007, 14(12), 792-798. https://doi.org/10.1016/j.phymed.2007.06.008
-
Trang, D.; Linh, V. C.; Lan, N. T. P. Antihyperglycemic, antioxidant, and antihyperlipidemic activities of extract from Coccinia grandis (L.) Voigt. leaves by methanol on alloxan induced hyperglycemic mice. Vietnam J. Biotechnol. 2018, 16(3), 485-493.
-
Basini, J.; Kumar, K.; Swetha, A.; Roshini, P.; Prathima, N.; Chandana, D. In-vivo antihyperlipidemic activity of ethanolic fruit extract of Coccinia grandis Linn. against dexamethasone induced hyperlipidemia. World J. Pharm. Res. 2020, 9(7), 1297-1306.
-
Chandimali, N.; Bak, S. G.; Park, E. H.; Lim, H.; Won, Y.; Kim, E.; Park, S.; Lee, S. J. Free radicals and their impact on health and antioxidant defenses: a review. Cell Death Discovery 2025, 11(1), 2278. https://doi.org/10.1038/s41420-024-02278-8
-
Chaudhary, P.; Janmeda, P.; Docea, A. O.; Yeskaliyeva, B.; Razis, A. F. A.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative stress, free radicals and antioxidants: potential crosstalk in the pathophysiology of human diseases. Front. Chem. 2023, 11, 1158198. https://doi.org/10.3389/fchem.2023.1158198
-
Leyane, T. S.; Jere, S. W.; Houreld, N. N. Oxidative Stress in Ageing and Chronic Degenerative Pathologies: Molecular Mechanisms Involved in Counteracting Oxidative Stress and Chronic Inflammation. Int. J. Mol. Sci. 2022, 23(13), 7273. https://doi.org/10.3390/ijms23137273
-
Jomová, K.; Raptová, R.; Alomar, S. Y.; Alwasel, S. H.; Nepovimova, E.; Kuča, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: chronic diseases and aging. Arch. Toxicol. 2023, 97(10), 2499-2574. https://doi.org/10.1007/s00204-023-03562-9
-
Tripathi, R.; Gupta, R.; Sahu, M.; Srivastava, D.; Das, A.; Ambasta, R. K.; Kumar, P. Free radical biology in neurological manifestations: mechanisms to therapeutics interventions. Environ. Sci. Pollut. Res. 2021, 29(41), 62160-62207. https://doi.org/10.1007/s11356-021-16693-2
-
Shankar, R.; Gnaneswari, K.; Devi, T.; Niharika, B. In-vitro anti-inflammatory, anti-oxidant and In-vivo anti-urolithiatic activities of Coccinia grandis fruit extract. GSC Adv. Res. Rev. 2024, 18(1), 124-132.
-
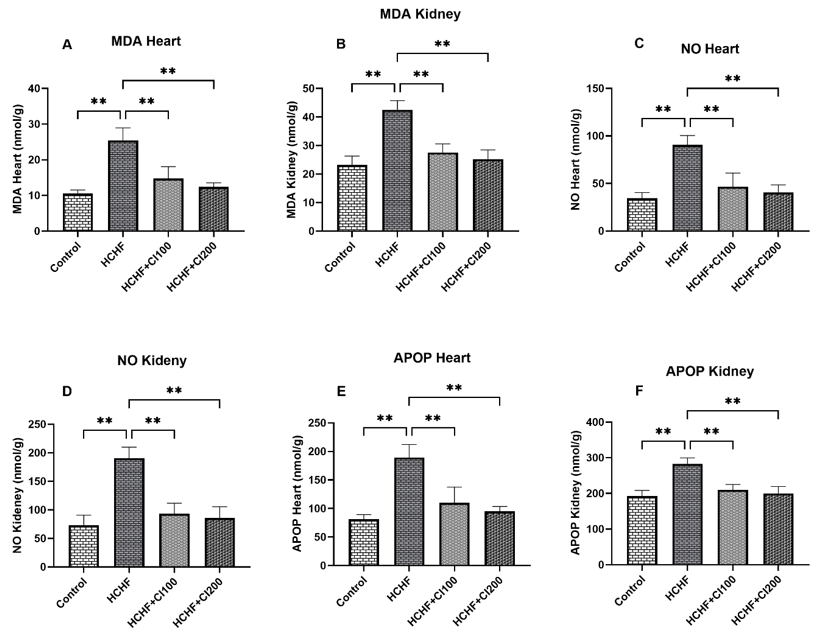
Banerjee, A.; Mukherjee, S.; Maji, B. Efficacy of Coccinia grandis against monosodium glutamate induced hepato-cardiac anomalies by inhibiting NF-kB and caspase 3 mediated signalling in rat model. Hum. Exp. Toxicol. 2021, 40(11), 1825-1851. https://doi.org/10.1177/09603271211010895
-
Umamaheswari, M.; Asokkumar, K.; Somasundaram, A.; Sivashanmugam, T.; Subhadradevi, V.; Ravi, T. k. Xanthine oxidase inhibitory activity of some Indian medical plants. J. Ethnopharmacol. 2007, 109(3), 547-551. https://doi.org/10.1016/j.jep.2006.08.020
-
Kumar, D.; Kumar, M. S. Evaluation of Anticonvulsant Activity of Hydro Ethanolic Extract of Coccinia grandis in Mice. Sch. Acad. J. Pharm. 2022, 11(9), 147-152. https://doi.org/10.36347/sajp.2022.v11i09.005
-
Gaul, S.; Leszczyńska, A.; Alegre, F.; Kaufmann, B.; Johnson, C. D.; Adams, L. A.; Wree, A.; Damm, G.; Seehofer, D.; Calvente, C. J.; povero,D.; Kisseleva, T.; Eguchi, A.; McGeough, M. D.; Hoffman, H. M.; pelegrin, P.; Laufs, U.; Feldstein, A. E. Hepatocyte pyroptosis and release of inflammasome particles induce stellate cell activation and liver fibrosis. J. Hepatol. 2021, 74(3), 674-686. https://doi.org/10.1016/j.jhep.2020.07.041
-
Gong, J.; Tu, W.; Liu, J.; Tian, D.-L. Hepatocytes: A key role in liver inflammation. Front. Immunol. 2023, 13, 1083780. https://doi.org/10.3389/fimmu.2022.1083780
-
Zhang, X.; Wu, X.; Hu, Q.; Wu, J.; Wang, G.; Hong, Z.; Ren, J. Mitochondrial DNA in liver inflammation and oxidative stress. Life Sci. 2019, 236, 116464. https://doi.org/10.1016/j.lfs.2019.05.020
-
Konishi, T.; Lentsch, A. B. Hepatic Ischemia/Reperfusion: Mechanisms of Tissue Injury, Repair, and Regeneration. Gene Expr. 2017, 17(4), 277-287. https://doi.org/10.3727/105221617X15042750874156
-
An, P.; Wei, L. L.; Zhao, S.; Sverdlov, D. Y.; Vaid, K. A.; Miyamoto, M.; Kuramitsu, K.; Lai, M.; Popov, Y. V. Hepatocyte mitochondria-derived danger signals directly activate hepatic stellate cells and drive progression of liver fibrosis. Nat. Commun. 2020, 11(1), 2206. https://doi.org/10.1038/s41467-020-16092-0
-
Lehmann-Werman, R.; Magenheim, J.; Moss, J.; Neiman, D.; Abraham, O.; Piyanzin, S.; Zemmour, H.; Fox, I; Dor, T.; Grompe, M.; Landesberg, G.; Loza, B.; Shaked, A.; Olthoff, K.; Glaser, B.; Shemer, R.; Dor, Y. Monitoring liver damage using hepatocyte-specific methylation markers in cell-free circulating DNA. JCI Insight 2018, 3(12), e120687. https://doi.org/10.1172/jci.insight.120687
-
Akhtam, R.; Nuraliyevna, S. N.; Kadham, M. J.; Mirzakhamitovna, K. S.; Tursunaliyevna, R. M.; Shakhnoz, K.; Shakhzod. T.; Otabek, B.; Baxtiyorovich, M. I.; Shakhboskhanovna, A. F.; Zulxumoroxon, B.; Isroilovna, I. M.; Khodji-Akbarovna, N. R. Biomarkers in liver regeneration. Clin. Chim. Acta 2025, 576, 120413. https://doi.org/10.1016/j.cca.2025.120413
-
Wei, C.; Wu, L.; Wu, Y.; Xu, C.; Hu, H.; Wang, Z. Selection and evaluation of quality markers (Q-markers) of vladimiriae radix extract for cholestatic liver injury based on spectrum-effect relationship, pharmacokinetics, and molecular docking. J. Ethnopharmacol. 2024, 329, 118151. https://doi.org/10.1016/j.jep.2024.118151
-
Yuan, H.; Liu, Z.; Chen, M.; Xu, Q.; Jiang, Y.; Zhang, T.; Suo, C.; Chen, X. Protein truncating variants in mitochondrial-related nuclear genes and the risk of chronic liver disease. BMC Med. 2024, 22(1), 234. https://doi.org/10.1186/s12916-024-03466-0
-
Staufer, K.; Huber, H.; Zessner-Spitzenberg, J.; Stauber, R.; Finkenstedt, A.; Bantel, H.; Weiss, T. S.; huber, M.; Starlinger, P.; Gruenberger, T.; Reiberger, T.; Sebens, S.; Mclntyre, G.; Tabibiazar, R.; Giaccia, A.; Zoller, H.; Trauner, M.; Mikulits, W. Gas6 in chronic liver disease-a novel blood-based biomarker for liver fibrosis. Cell Death Discovery 2023, 9(1), 258. https://doi.org/10.1038/s41420-023-01551-6
-
Dallio, M.; Romeo, M.; Cipullo, M.; Ventriglia, L.; Scognamiglio, F.; Vaia, P.; Ladanza, G.; Coppola, A.; Federico, A. Systemic Oxidative Balance Reflects the Liver Disease Progression Status for Primary Biliary Cholangitis (Pbc): The Narcissus Fountain. Antioxidants 2024, 13(4), 387. https://doi.org/10.3390/antiox13040387
-
Wang, K.; Fang, Q.; He, P.; Tu, Y.; Liu, Z.; Li, B. Unveiling the potential of selenium-enriched tea: Compositional profiles, physiological activities, and health benefits. Trends Food Sci. Technol. 2024, 145, 104356. https://doi.org/10.1016/j.tifs.2024.104356
-
Vadivu, R.; Krithika, A.; Biplab, C.; Dedeepya, P.; Shoeb, N.; Lakshmi, K. S. Evaluation of Hepatoprotective Activity of the Fruits of Coccinia grandis Linn. Int. J. Health Res. 2008, 1(3), 163-168. https://doi.org/10.4314/ijhr.v1i3.55366
-
Yerramilli, V.; Singh, M.; Singh, I.; Nagar, L.; Singh, J. Hepato-restorative Activity of Methanolic Extracts of Coccinia grandis L. Voigt. in CCl4 - Intoxicated Rats. Pharmacogn. J. 2024, 16(5), 1165-1171. https://doi.org/10.5530/pj.2024.16.178
-
Kumar, P.; Sivaraj, A.; Elumalai, E. K.; Kumar, B. S. Carbon tetrachloride-induced hepatotoxicity in rats - protective role of aqueous leaf extracts of Coccinia grandis. Int. J. PharmTech Res. 2009, 1(4), 1612-1615.
-
Chidambaram, R.; Devi, R.; Selvi, R.; Alagendran, S.; Bhaskar, A. Protective effect of Coccinia grandis [L] against (Diethylnitrosamine) DEN-induced Hepatotoxicity in Wistar Albino Rats. Int. J. Pharm. Sci. Rev. Res. 2016, 38(1), 160-165.
-
Banerjee, A.; Mukherjee, S.; Maji, B. Coccinia grandis alleviates flavor-enhancing high-lipid diet induced hepatocellular inflammation and apoptosis. J. Food Biochem. 2022, 46(5), e14092. https://doi.org/10.1111/jfbc.14092
-
Kundu, M.; Mazumder, R.; Kushwaha, M. Evaluation of hepatoprotective activity of ethanol extract of Coccinia grandis (L.) Voigt. leaves on experimental rats by acute and chronic models. Orient. Pharm. Exp. Med. 2012, 12(2), 93-97. https://doi.org/10.1007/s13596-012-0057-3
-
Aljehany, B. M.; Abduljawad, E. A. Protective Effect of Coccinia grandis on Cyclophosphamide Induced Liver Damage. Am. J. Res. Commun. 2018, 6(2), 28-39.
Author Affiliation
1Department of Pharmaceutical Sciences, North South University, Dhaka, Bangladesh
2Montclair State University, Montclair, NJ, USA
ARTICLE INFO
Dr. Nasrin Akhter , Assistant Professor, Department of Pharmacy, Independent University of Bangladesh, Dhaka, Bangladesh